Motlagh, P. Y., Khataee, A., & Hassani, A. (2024). Preparation of La-doped NiAl-LDH with boosted electron transfer for the enhanced photocatalytic activity of tetracycline: Performance evaluation, degradation pathway, and mechanism insight. Journal of Alloys and Compounds, 1008, 176448.
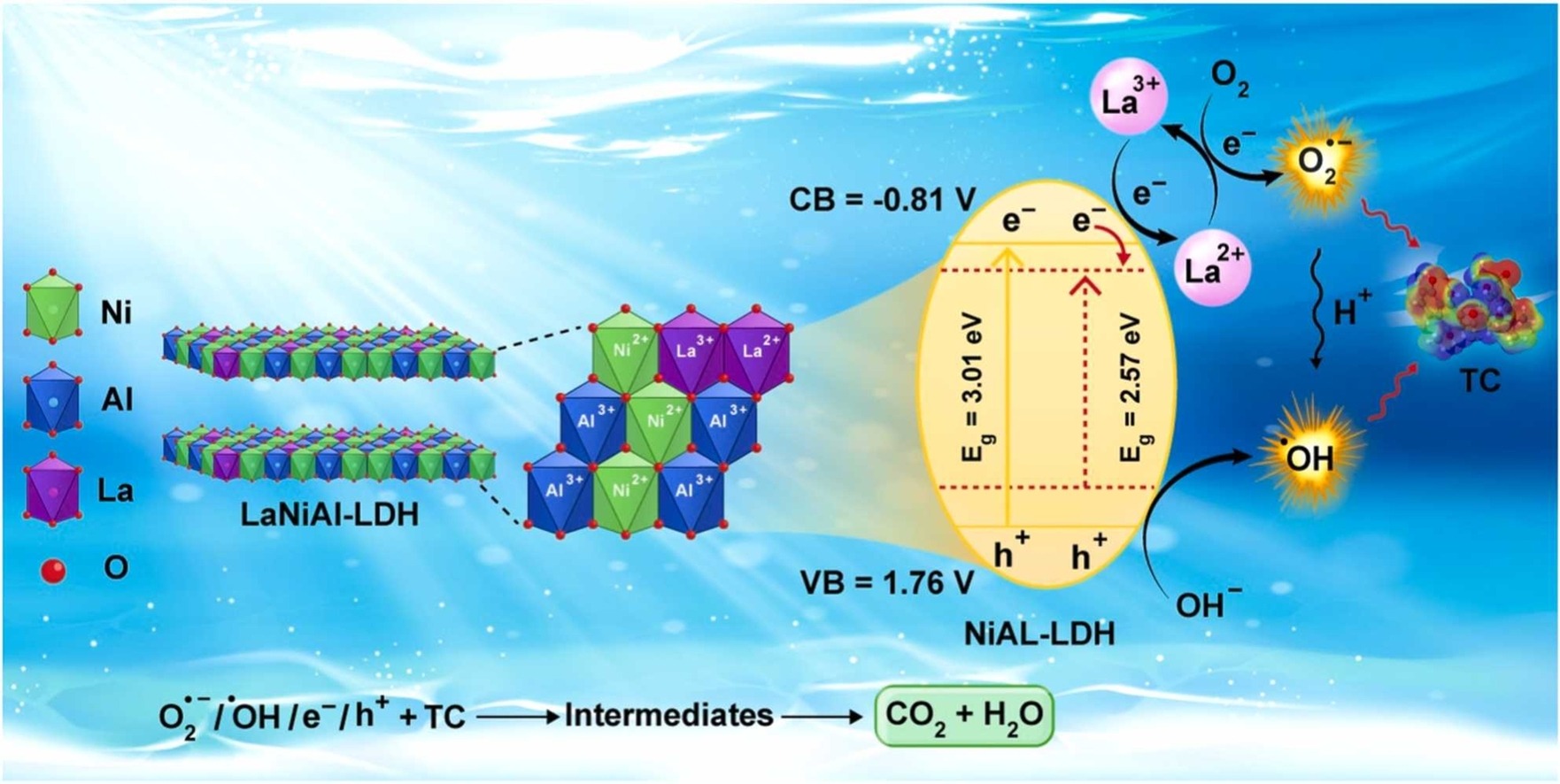
The study focuses on developing an efficient photocatalysis-based system for removing antibiotics, specifically tetracycline (TC), from wastewater. A novel La-doped NiAl-layered double hydroxide (LaNiAl-LDH) photocatalyst was synthesized via a simple chemical co-precipitation-hydrothermal method. Advanced characterization techniques, including XRD, FTIR, and photoluminescence (PL) analysis, confirmed the successful synthesis and properties of the material. The addition of La into the NiAl-LDH structure reduced the band gap from 3.01 eV to 2.57 eV, significantly enhancing photocatalytic activity. Among various dopant percentages, 1% LaNiAl-LDH showed the best performance, achieving 84.85% degradation of TC (10 mg/L) under visible light within 90 minutes, compared to 71.52% for undoped NiAl-LDH.
The enhanced activity was attributed to La sites acting as electron-transfer mediators, facilitating effective separation of photogenerated electron-hole pairs. Morphological studies revealed the formation of nanosheets in the LDH structure, contributing to the material’s high surface area and catalytic efficiency. The degradation process was further supported by the detection of reactive oxygen species, such as •OH radicals, which were identified as the main active species through scavenger experiments. Operational variables, including photocatalyst dosage, initial TC concentration, pH, and lamp power, were optimized to maximize the degradation rate.
In addition to demonstrating high photocatalytic efficiency, the LaNiAl-LDH photocatalyst exhibited good reusability, maintaining 72% degradation efficiency after six cycles, with negligible metal leaching and structural stability. The proposed degradation pathway highlighted the breakdown of TC through the dissociation of functional groups and benzene ring opening. Moreover, the catalyst showed resistance to interference from various anions and effectively degraded pollutants in real wastewater.
This research highlights the potential of La-doped LDH-based photocatalysts for sustainable and efficient antibiotic removal, offering a promising solution for wastewater remediation and environmental protection.
Dr. Aydin Hassani is an associate professor in the Department of Materials Science and Nanotechnology Engineering at Near East University. For those interested in pursuing research or collaborations in advanced oxidation processes, water and wastewater treatment, photocatalysis, nanomaterials, and materials chemistry, Dr. Aydin Hassani ([email protected]) is available for inquiries. With expertise in cutting-edge nanotechnology and environmental materials science, Dr. Hassani provides valuable insights and guidance for advancing work in these innovative fields.
Abstract
Developing a photocatalysis-based system is an effective strategy for removing antibiotics. Herein, La-doped NiAl-layered double hydroxides (LaNiAl-LDH) photocatalyst was synthesized using a chemical co-precipitation-hydrothermal method. The prepared samples were characterized by various advanced analyses and investigated for the photocatalytic degradation of tetracycline (TC). The crystalline phase and functional groups of the synthesized samples were successfully confirmed by XRD and FTIR analyses. By doping La into NiAl-LDH structure, the band gap was reduced from 3.01 eV to 2.57 eV, indicating higher photocatalytic activity of 1% LaNiAl-LDH. The surface morphology of the synthesized samples shows the formation of nanosheets in the structure of LDH. Under the optimum status ([TC]0 = 10 mg/L, [1% LaNiAl-LDH] = 0.2 g/L, and pH = 6), the photocatalytic activity of 1% LaNiAl-LDH (84.85%) was higher than NiAl-LDH (71.52%) within 90 min reaction. This difference can be attributed to the presence of La sites in 1% LaNiAl-LDH which act as electron-transfer mediators, leading to a significant improvement in the separation of photogenerated charge carriers. The effect of the addition of various scavengers was evaluated. Moreover, using the o-phenylenediamine (OPD), and photoluminescence (PL) analysis, the formation of •OH radicals was supported. The higher charge-transfer efficiency of 1% LaNiAl-LDH was confirmed by photoelectrochemical analysis. Furthermore, the reusability, stability, and degradation pathway of the as-synthesized nanocomposite were systematically examined. This work presents guidance for applying doped LDH-based materials in photocatalysis and promising applications in wastewater remediation.